Interview with IMAG’IC
[Interview with]
Thomas Guilbert, Research Engineer

Hi Thomas, could you introduce yourself and give us a quick understanding of what Imag’IC is?
 Hi Oxxius, I am 37yo, I have a PhD in physics (non-linear microscopy), I am research engineer at IMAG’IC, the photonic microscopy core-facility which is headed by Pierre Bourdoncle for the technical aspect and Florence Niedergang at the scientific direction. IMAG’IC is one of the 10 facilities of Institut Cochin, a biomedical research center located downtown Paris. Specifically, I am in charge of optic development, multiphotonic experiments and quality control of microscopes. I am also involved in professional networks dedicated to light microscopy as the CNRS RTMFM for France and QUAREP internationally.
Hi Oxxius, I am 37yo, I have a PhD in physics (non-linear microscopy), I am research engineer at IMAG’IC, the photonic microscopy core-facility which is headed by Pierre Bourdoncle for the technical aspect and Florence Niedergang at the scientific direction. IMAG’IC is one of the 10 facilities of Institut Cochin, a biomedical research center located downtown Paris. Specifically, I am in charge of optic development, multiphotonic experiments and quality control of microscopes. I am also involved in professional networks dedicated to light microscopy as the CNRS RTMFM for France and QUAREP internationally.
At IMAG’IC, our 4 members team manages 15 acquisition systems (widefield, confocal, super-resolution (PALM, sptPALM, 3D STORM, 3D STED), multiphotonic intravital TPEF/SHG/THG, TIRF, FLIM, flow cytometry imaging…) and 3 analysis stations for image processing. Any person working in a public laboratory or a private company can benefit assistance, training and even become autonomous to work on all the microscopes managed by the facility. We have around 300 users per year.
What kind of research do you carry out ?
The Institut Cochin represents 38 different research teams divided in 3 departments (Endocrinology, Metabolism and Diabetes, Development, Reproduction and Cancer, Infection, Immunity and Inflammation), which means a very large diversity of biological samples. The specificity of our core-facility is to provide high-end microscopy systems in many different biosafety levels (BSL 1, 2 & 3). This means that we can image fixed as well as alive human samples, infected with bacteria and viruses like HIV, hepatitis virus or SARS-CoV 2.
Could you present us your spinning disk set up ?
In our BSL3 environment, we provide an inverted microscope equiped with a Yokogawa CSU-X1 spinning-disk to assess confocal optical sectionning at high magnification. Heating chamber and CO2 supply allow us to provide a good environment for the proper development of cells under imaging conditions. Images are acquired through either a CCD or an EMCCD camera, depending on the dynamic time we want to monitor and the resolution we want to reach. The whole system is driven with Metamorph software.
We needed more powerful and stable light sources to ensure good performances to monitor biological events that can last several days. The OXXIUS combiner gathering 4 laser lines (405, 488, 561, 638nm) totally makes it.
ZOOM ON
|
Do you already have some first pictures to communicate ?
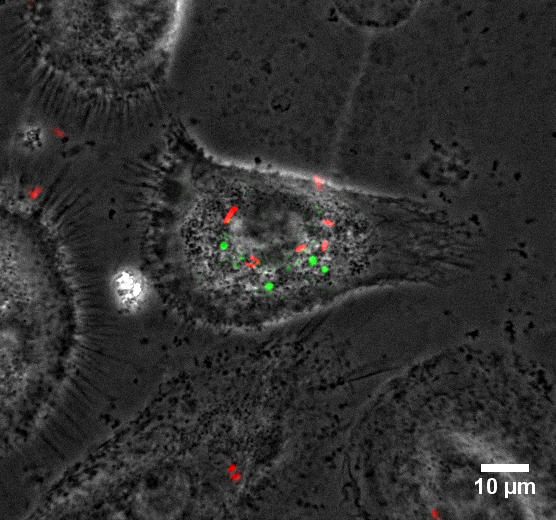
Credits : Gabrielle Lê-Bury & Florence Niedergang, Institut Cochin
Macrophages are recognised as reservoirs for HIV-1, which modifies the functions of these cells and makes them less capable to clear bacteria. How opportunistic bacteria take advantage of the HIV-1-infected macrophages is studied here: primary human macrophages were infected for 8 days with HIV-1 expressing GFP (in green) before infection with Salmonella Typhimurium expressing DsRed (in red). Live imaging was then performed for up to 6h at 37°C in the BSL3 laboratory.

